Story behind the paper: The enigmatic persistence of dissolved organic matter in the oceans
It's now about one year that we published a perspectives paper on the enigmatic persistence of dissolved organic matter in the ocean. Here's the story behind the paper. It is a story about the properties and transformation of marine Dissolved Organic Matter (DOM), one of the most mysterious matter pools in the earth system.
Background - the marine organic carbon cycle and the microbial loop
It begins in the sunlit upper ocean. Myriads of phytplankton cells use the energy of the sun to fix atmospheric CO2 and transform it into organic carbon. This is a tremendous process, it contributes to half of the Earth's oxygen production and the generated phytoplankton biomass provides the base for basically the whole marine food web. Most of the produced organic carbon is later on remineralized by respiration and released as CO2 back into the atmosphere, thus completing the marine carbon cycle (Fig. 1).
A small fraction of the fixed organic carbon, however, escapes rapid remineralization and is transported into the vast basins in the dark realm of the ocean. This can be in the form of sinking biogenic particles which are eventually buried in the sea floor and thus are removed from the climate system (biological pump). Or, it can be in the form of dissolved molecules (DOM) that can store organic carbon for long times in the water column and thus also, at least temporarily, rendering it climate neutral.
This dissolved organic carbon is produced by aquatic organisms (predominantly by phytoplankton cells in the surface ocean) that continuously release organic molecules into the surrounding water, either as waste-products during metabolism or set free upon death or lysis of cells. In both cases, the organic molecules accumulate as DOM in the water column and are transported by ocean circulations into the deep sea (Fig. 1).
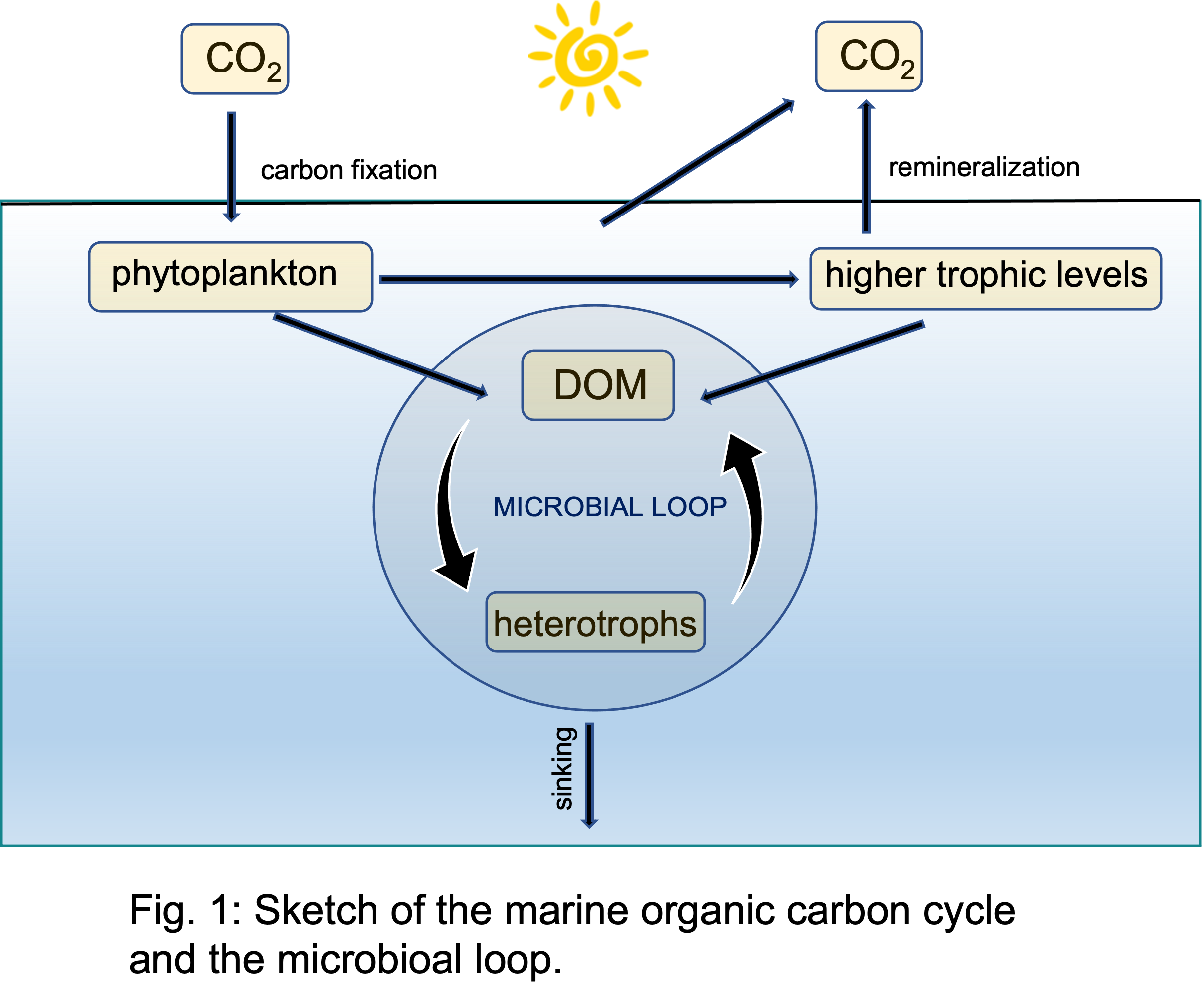
The journey of organic molecules does not stop there. Reduced carbon in organic molecules is a welcome source of energy for heterotrophic microorganisms, such as bacteria and archaea. The accumulated DOM thus provides the food source for a rich microbial community in the ocean. The resulting biomass is enormous. Every teaspoon of seawater contains on average a million bacterial cells that are living on DOM. Accumulate this over the whole volume of earth's oceans to get an idea about the shear size of the standing microbial biomass in the ocean.
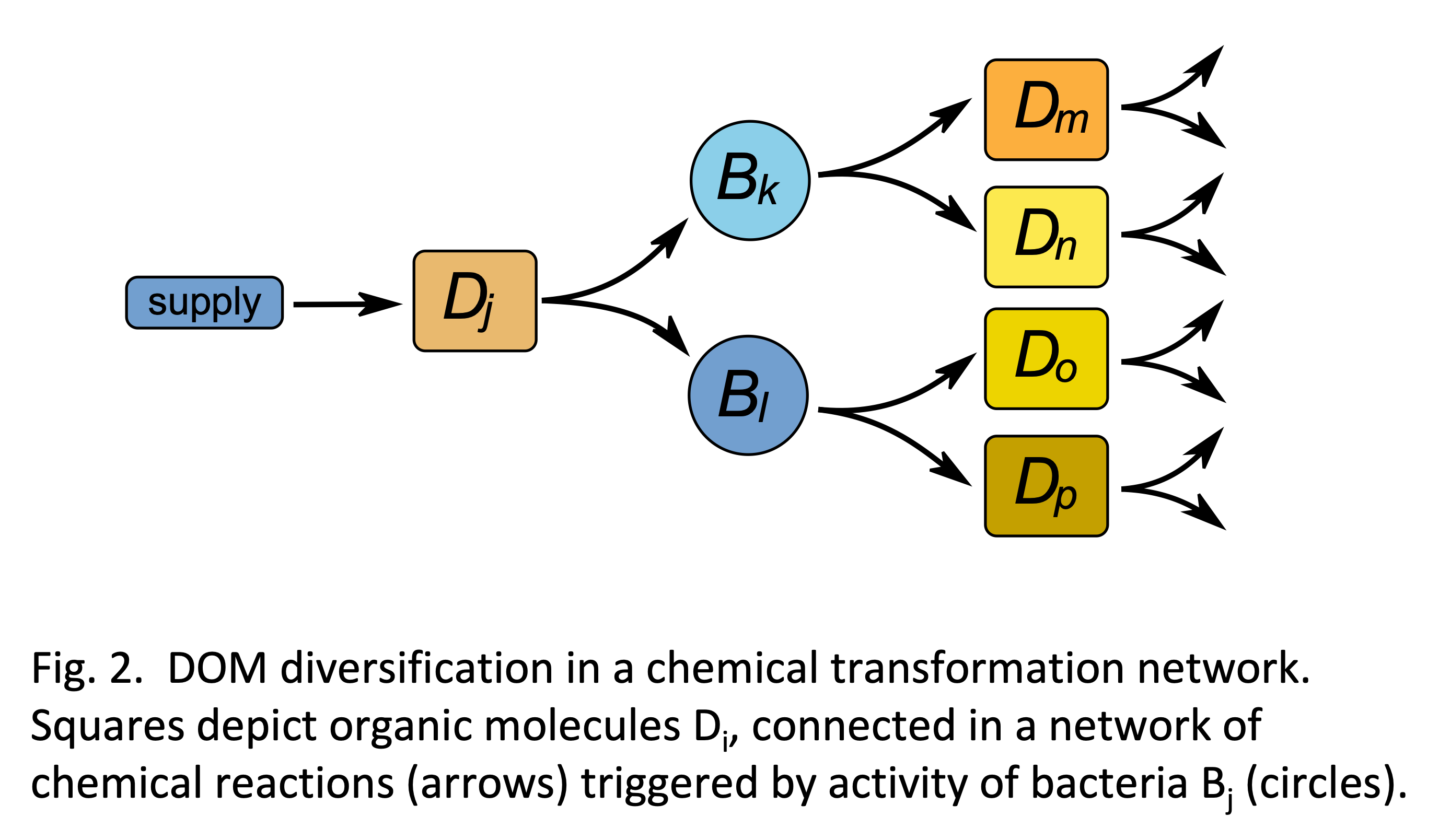
Now comes the important part: all these microbes, again, continuously release DOM. Typically thereby new organic compounds are produced, which over sufficient long time scales accumulate in the water column and then become food for other micro-heterotrophs, specialized on these compounds (Fig. 2). This cross-feeding process results in a diversification of the externally supplied DOM compounds, as more and more ‘empty niches’ in chemical space are occupied, in total, yielding a continuous cycling of carbon in a complex interaction network of microbial uptake, DOM release, and cross-feeding (the microbial loop).
Three remarkable properties of DOM
As we argue in our perspectives paper, the tight interaction network in the microbial loop could be directly responsible for three remarkable properties of marine DOM, namely 1) its huge diversity, 2) its huge size, and 3) its huge age.
-
DOM is a highly diverse chemical mixture, constituting of probably more than 100,000, maybe millions, of chemical compounds. This chemical diversity is not tractable at a molecular level by analytical chemistry. Thus, the molecular composition and exact diversity of DOM is largely unknown. Conversely, the high diversity has the consequence that the concentration of individual DOM compounds must be extremely small - with implications on bacteria-DOM uptake rates (dilution effect).
-
DOM constitutes one of the largest pools of reduced carbon in the biosphere. It stores an amount of approx. 700 Petagram carbon, which is comparable in size to the atmospheric CO2 inventory and contains more carbon than Earth’s marine and terrestrial biota combined. This is relevant for the potential carbon storage in the future ocean: due to its sheer size minor changes in DOM stability could impact atmospheric CO2 and thus the Earth heat budget.
-
DOM is old, with an estimated lifetime of several thousand years. DOM age is, however, rather heterogeneously distributed among different compounds. Most of the supplied DOM, called labile DOM, is quickly consumed within minutes to days after production. Labile DOM does not accumulate in the ocean, and thus occurs only in low concentrations, owing to the rapid microbial turnover, and it is only present in productive surface waters. In contrast, a small fraction of the supplied DOM, called refractory DOM, escapes immediate utilization and accumulates in the ocean over millennia. Refractory DOM makes up the bulk of the DOM pool and is rather homogenously distributed in the world oceans.
Deep-sea DOM paradox: starving microbes in a sea of substrate
The mere existence of refractory DOM is a geochemical enigma because such a huge fraction of persisting DOM simply should not exist. Given the fact that heterotrophic microbial life in the deep ocean is carbon limited and that microorganisms are surrounded by an enormous DOM pool, it is a puzzle why microorganisms fail to utilize this energy source. Thus, the paradox is why do 700 Pg of dissolved organic carbon persist in the ocean for thousands of years, while heterotrophic microbial life in the deep sea is carbon limited?
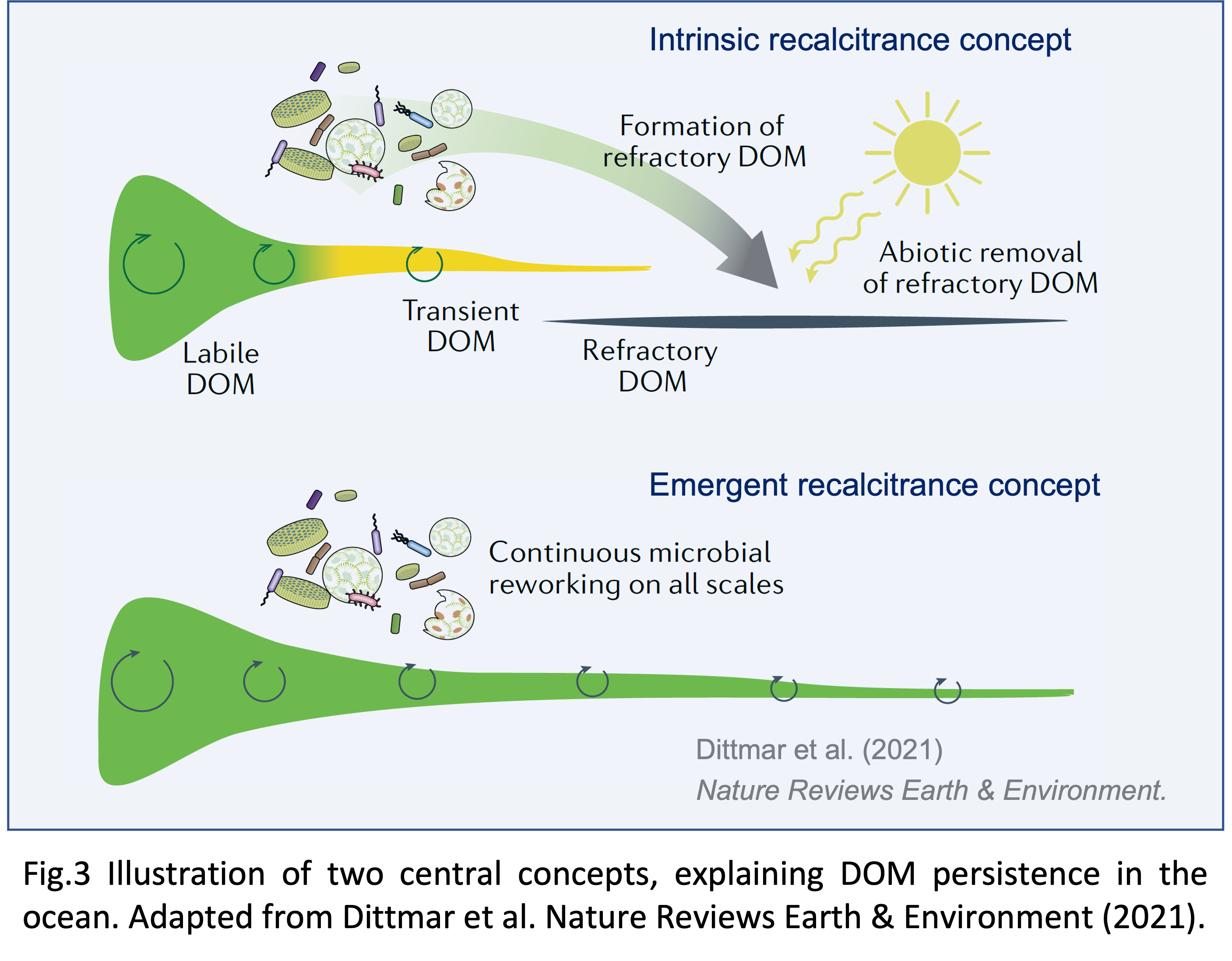
In our perspectives paper we present the two main hypotheses that have been put forward to explain DOM persistence: the intrinsic recalcitrance concept and the emergent recalcitrance concept (Fig. 3).
Intrinsic vs emergent recalcitrance
The intrinsic recalcitrance concept (see e.g. Jiao et al., 2010) is the classical explanation for DOM persistence. According to this concept, labile and refractory DOM correspond to two different molecule types that differ in their specific intrinsic molecular properties. Labile DOM is easily taken up by microbes, it thus occurs only in small concentrations and by microbial processing is converted into the refractory form of DOM. This consists of recalcitrant molecules that are highly resistant to microbial degradation and so can accumulate in the ocean to large age and concentration (Fig 3, top).
The intrinsic recalcitrance is a very natural and plausible explanation. The only problem is that there are indications that this explanation simply may not be true, or at least not entirely. First, the presence of truly non-degradable molecules is hard to conceive, as we don’t know any biomolecules that cannot be broken down in one way or the other by microbes. Second, there is experimental evidence that micro-heterotrophs are able grow on experimentally concentrated deep-sea DOM (Arieta, 2015), which is in striking contrast to the assumption of non-degradable molecules.
These problems led some researchers to look for other explanations of DOM persistence. The most promising candidate is the emergent recalcitrance concept (Fig 3, bottom) which does not postulates different molecule types. In contrast, according to this concept, all DOM compounds can in principle be decomposed by microbes. As a consequence, following the mechanism shown in Fig. 2, DOM is continuously transformed and diversified by microbial activity on all scales. As DOM diversity increases and concentrations decline, degradation eventually slows down, yielding the long life-times of the accumulating DOM compounds.
Origin of irreversibility in the labile-to-refractory transformation
One way to better understand the origin of DOM persistence is to view it with respect to the reversibility of the labile-to-refractory DOM transformation. What force is acting to pump organic molecules against the existing concentration gradient from low-concentrated labile DOM to highly-concentrated refractory DOM? The two above mentioned recalcitrance concepts give strikingly different answers to this question.

According to the intrinsic recalcitrance concept, we can think of the formation of refractory DOM as a simple chemical reaction from a source pool of labile DOM towards a product pool of refractory DOM (Fig. 4). The persistence of DOM is explained by proposing that this reaction is uni-directional, that is, the backward reaction rate is supposed to be zero or negligibly small (as recalcitrant molecules cannot be transformed by microbes). Thus, irreversibility arises at the level of a single reaction, which naturally yields an equilibrium state with a low concentration of the substrate (here, labile DOM) and a high concentration of the product (here, refractory DOM.)
Interlude: molecular entropy
Before discussing the origin of irreversibility in the emerging recalcitrance concept, let us first make a small excursion into thermodynamics to recap the notion of entropy. Entropy is a central concept in thermodynamics and information theory, providing a precise measure of uncertainty about the state of a physical system. Assume that our knowledge about the state is described by a probability distribution $p$. Applied to a chemical mixture of DOM molecules, in the simplest case, this could describe the relative concentration $p_i$ of different molecule types, which are normalized as $\small \sum_i p_i=1$.
Following Shannon (1948), the (information) entropy is given as $\small H=\sum_i p_i \log{p_i}$, which we denote in our context as the molecular entropy $\small H$ of the DOM mixture. The information entropy is frequently used as a measure of diversity (the Shannon-Wiener index); thus, a configuration of high molecular entropy corresponds to high molecular diversity.
The molecular entropy can also be thought of as a measure of the likelihood of the system to be in one particular physical or geochemical state as opposed to another. For this, it is helpful to distinguish between the macrostate (here, the measurable geochemical state of the ocean) and the microstate (the non-measurable specific configuration of molecules into compounds). Since a large number of microstates are consistent with the same macrostate, the likelihood to observe a certain geochemical state is proportional to the number of corresponding microstates, that is, the number of ways that the system can be in that measurable state. The macrostate that has the largest number of microstates associated to it is the most probable state and has the highest entropy.
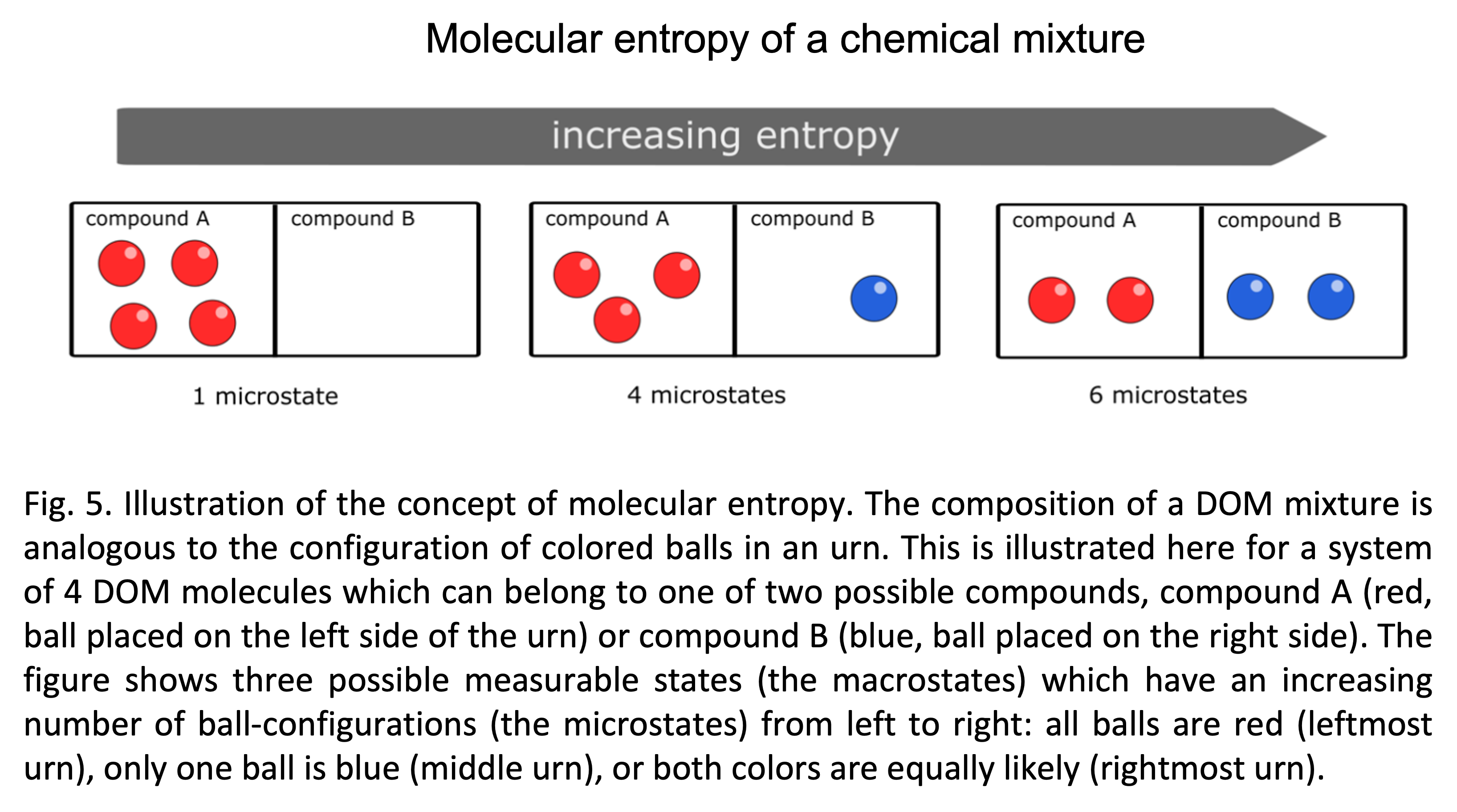
To illustrate these ideas, consider a simple mixture of four molecules that each can belong to one of two DOM compounds. The number of microstates can be counted in analogy to the number of ways how four colored balls can be placed in an urn (Fig. 5). There is only one configuration (leftmost urn in the figure) in which all four balls are red (i.e., all molecules belong to compound A). Thus, the macrostate of dominance of A ($\small p_A=1$ and $\small p_B=0$) is associated to only one microstate and has a low entropy with Shannon index $\small H= - 1 \log(1)=0$. The urn in the middle shows a configuration where one ball is blue and the others are red. Assuming the balls (or molecules) are distinguishable, this macrostate ($\small p_A=3/4$ and $\small p_B=1/4$) can be achieved via four possible configurations (each of the four balls might be blue) and accordingly has higher entropy, with a Shannon index $\small H= - 0.75 \log(0.75) -0.25 \log(0.25)\approx 0.56$. Finally, in the most likely macrostate (rightmost urn in Fig. 5) all ball colors are equally likely ($\small p_A=1/2$ and $\small p_B=1/2$). This state can be achieved in 6 configurations because there are $\small \binom{4}{2} = 6$ possibilities to choose two balls out of the set of four. Thus, the state of equal partition has the largest number of microstates associated to it and has the highest molecular entropy $\small H= - 0.5 \log(0.5)-0.5 \log(0.5)= \log(2)\approx 0.69$. While in this simple example the number of microstates differed not very much between low and high entropy states, this difference rapidly becomes magnified with increasing numbers of balls and colors.
Origin of irreversibilty in the emergent recalcitrance concept
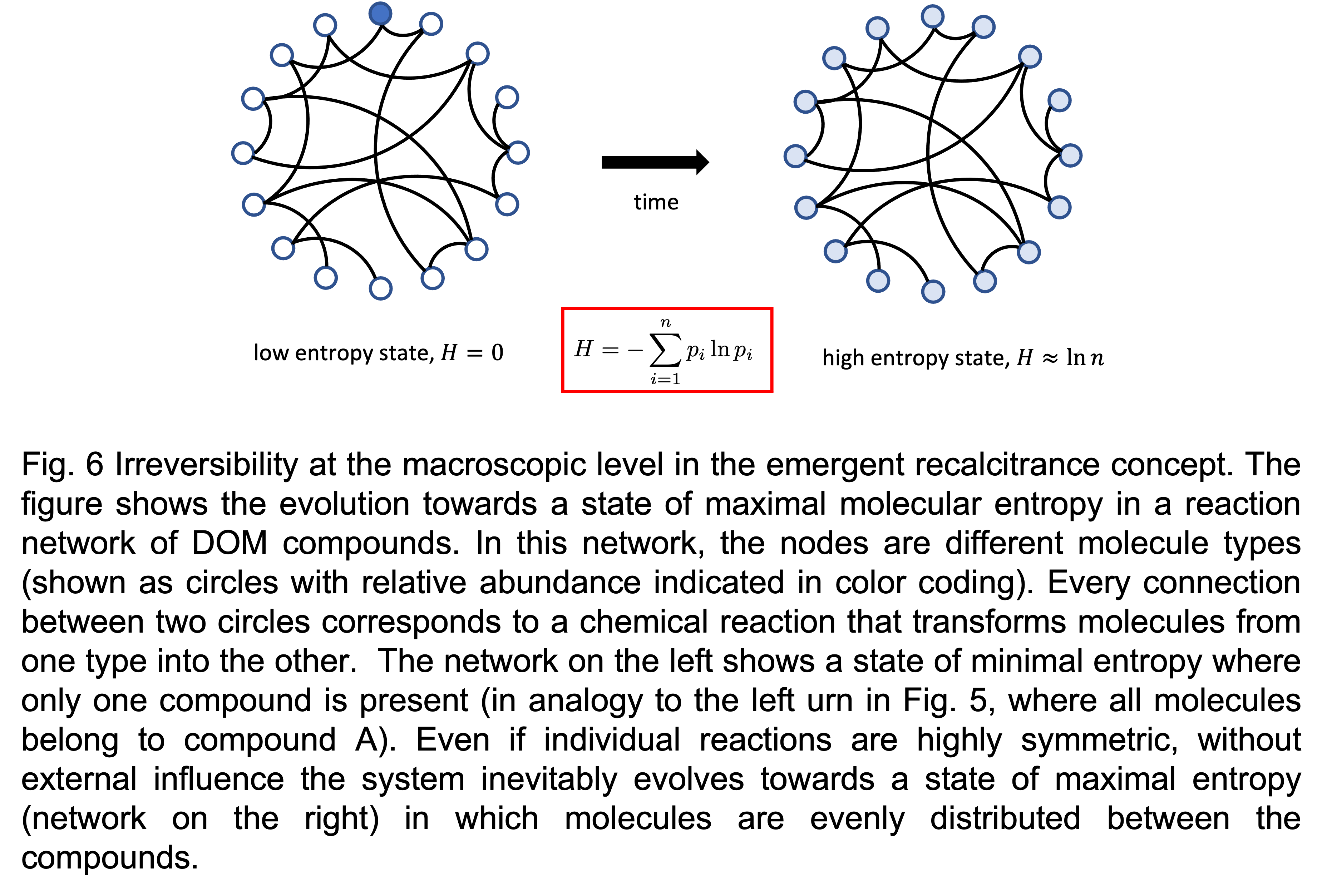
According to the second law of thermodynamics, entropy tends to increase in a closed system as the macrostate evolves towards more likely configurations. This allows to better understand the origin of irreversibility in the emergent recalcitrance concept. In the context of DOM, organic molecules are connected in a network of possible chemical reactions mainly triggered by microbial activity, transforming molecules between different compounds (Fig. 6). At the level of single reactions this transformation may be symmetric if forth and back reactions have similar rates. But at the level of the whole chemical mixture this symmetry is broken and the system will unidirectionally organize over time in such a way as to maximize its molecular entropy.
Consider, for example, the situation during an algal bloom in the surface ocean. All DOM molecules are concentrated into a few compounds of high concentration (shown as a single compound in Fig. 6 left), while all other compounds have concentration zero, $\small p_i=0$. This yields a configuration of low molecular entropy $\small H\approx 0$, as all molecules are concentrated in a few compounds. With ongoing aging and microbial processing of DOM new molecule types are created. Due to the complexity and apparent ‘randomness’ of the reaction network, the DOM molecules automatically explore all possible pathways in the network, and thus, by fundamental laws of thermodynamics, the system evolves towards a stationary distribution of maximal chemical entropy.
In this way, at the macroscopic level, a time arrow naturally emerges: the undirected chemical turnover due to microbial activity paradoxically drives the molecular composition of an unperturbed water mass towards a state of maximal entropy and, thus, molecular diversity. In this state, the low diversity of externally supplied DOM has been redistributed among the astronomically large ensemble of organic compounds that are stable in the water column and can possibly be produced by enzymatic activity. This huge diversity and the consequential low concentration of each individual compound limit microbial degradation rates and thus explain the long lifetime and huge standing stock of DOM in the ocean. A prerequisite for this mechanism is that all organic compounds can indeed be processed by microbial activity, that is, the emergent recalcitrance hypothesis.
So, which concept is right?
The persistence of marine DOM is currently a controversially debated topic, as both the intrinsic and the recalcitrance concepts provide in itself consisting pictures about the mechanisms at work. In the end, the debate will probably settle on a middle-ground, as usual with such battles in science. Both, the intrinsic and emergent recalcitrance concept will have their place, and in all likelihood both concepts will be important for understanding the structuring of DOM transformation.
Obviously, not all DOM transformation reaction rates can be equal. Thus, a disparity of reaction rates, as proposed by the intrinsic recalcitrance concept, will probably play an important role. One the other hand, there is overwhelming evidence that microbial cross-feeding and large diversification of DOM is a real thing. Thus, full understanding will also not be possible without embracing the concepts proposed by the emergent recalcitrance concept.
The question for the future will be how these concept can be combined and reconciled with each other. Here in all likelihood mathematical modelling will be very important. Due to the high molecular diversity in the microbial loop and the associated extremely low concentrations of single compounds, details about the fine structure of DOM-microbe interactions will be hard, if not impossible, to measure in the field. In contrast, studying the quantitative consequence of different mechanistic hypotheses in highly diverse interacting networks is just the challenge where mathematical modelling shines.
The connection to complex systems theory
In this sense, the interactions in the ocean microbiome provides a new frontier for complex systems theory. In this emerging field well-known concepts of theoretical ecology will have to be redefined. For example, while resource competition theory was traditionally defined in terms of competition for two essential ressources, in the microbiome we have to deal with competition of a highly diverse community of consumers for thousands and maybe millions of resources. Not surprisingly, in the last years many modelling studies concerning the interactions of microbial consumers with metabolites have been published, e.g., Butler & O‘Dwyer (2018), (2020); Goldford et al. Science (2018); Marsland et al. (2019); Niehaus et al (2019); Ona & Kost (2022).
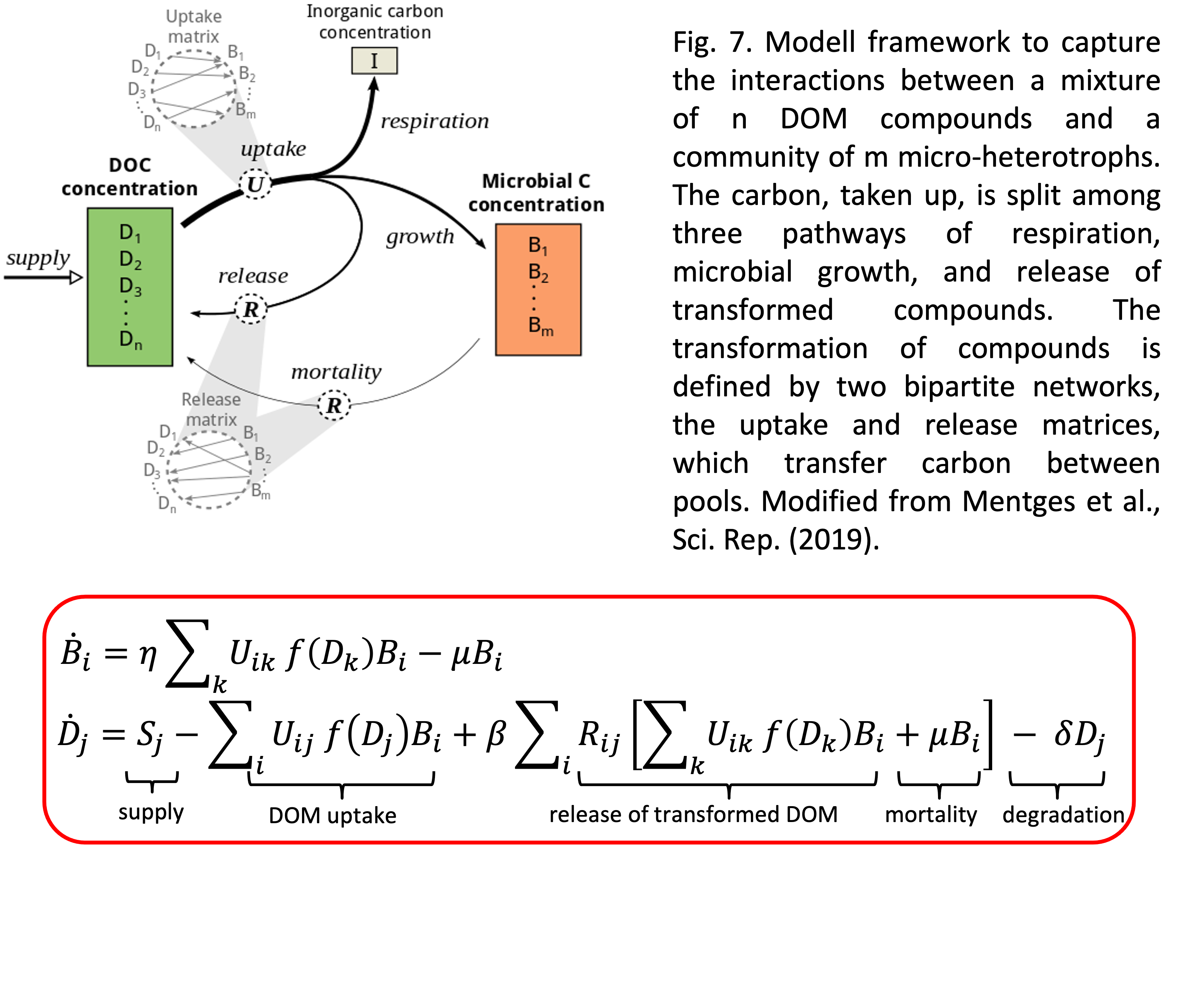
My own contribution to this topic (together with colleagues from the ICBM) was the development of a conceptual model how such an interaction network can be modelled in the simplest conceivable way (Mentges et al. 2019). The model framework is shown in Fig. 7 and basically describes the transformation of DOM in two bipartite networks of DOM uptake and release between DOM compounds and microbial consumers. The main finding was that structurally-recalcitrant molecules are not required in the model to explain either the amount or longevity of DOC. But most importantly, our model sets the ground for further investigations in that directions (see for example our follow-up studies Mentges et al. 2020, Lücken et al. 2022). In any case, these are interesting times for modellers in the field of microbiology and probably in the next few years we will see much progress in unravelling the mysteries of marine DOM.